
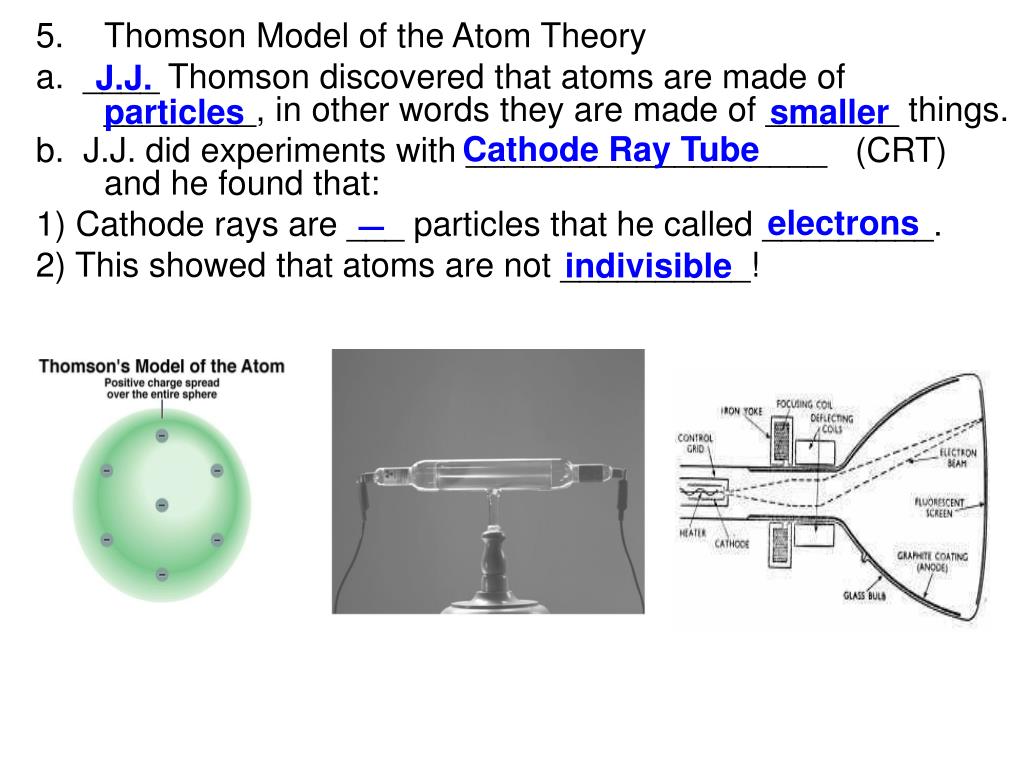
The transfer occurs due to the application of a voltage in vacuum. Cathode rays form when electrons emit from one electrode and travel to another. Thomson determined that charged particles much lighter than atoms, particles that we now call electrons made up cathode rays.

Cathode rays played a critical role in unlocking this mystery. Yet until Thomson, no one had determined what these might be. Prior to the discovery of the electron, several scientists suggested that atoms consisted of smaller pieces. What is a cathode ray tube and why was it important?
Jj thomson cathode ray tube experiment without labels full#
The proton and the neutron would soon follow as the full structure of the atom was discovered. For instance, the discovery of the electron was vital to the development of chemistry today, and it was the first subatomic particle to be discovered. Although he received the Nobel Prize in physics and not chemistry, Thomson’s contributions to the field of chemistry are numerous. Thomson did most of this work while leading the famed Cavendish Laboratory at the University of Cambridge. In addition to this work, Thomson also performed the first-ever mass spectrometry experiments, discovered the first isotope and made important contributions both to the understanding of positively charged particles and electrical conductivity in gases. Thompson made the switch to physics a few years later and began studying the properties of cathode rays.

Thompson was born in December 1856 in Manchester, England and was educated at the University of Manchester and then the University of Cambridge, graduating with a degree in mathematics. JJ Thomson was an English physicist who is credited with discovery of the electron in 1897. Learn all about the discovery, the importance of the discovery, and JJ Thomson in this tutorial article. JJ Thomson made the discovery using the cathode ray tube. The discovery of the electron was an important step for physics, chemistry, and all fields of science. This rule helps predict the major product in such reactions.Concept Introduction: JJ Thomson and the Discovery of the Electron Markovnikov's rule states that in the addition of a polar reagent (HX) to an alkene, the hydrogen atom (H) will attach to the carbon with the greater number of hydrogen atoms, and the halogen atom (X) will attach to the carbon with the fewer number of hydrogen atoms. This model was later replaced by the Rutherford's nuclear model and the Bohr's model, which further refined our understanding of atomic structure. Thomson's discovery of the electron led to the proposal of the "plum pudding model" of the atom, in which negatively charged electrons were embedded in a positively charged sphere. He calculated the charge-to-mass ratio of these particles and concluded that they were much lighter than atoms, leading to the discovery of the electron.īefore Thomson's experiment, the atom was considered to be indivisible and electrically neutral. He applied an electric field and a magnetic field perpendicular to the path of the cathode rays and found that the rays were deflected, indicating that they were negatively charged particles. He observed that a beam of particles, called cathode rays, was emitted from the negatively charged cathode and traveled towards the positively charged anode. Thomson passed an electric current through a glass tube containing gas at low pressure.


 0 kommentar(er)
0 kommentar(er)
